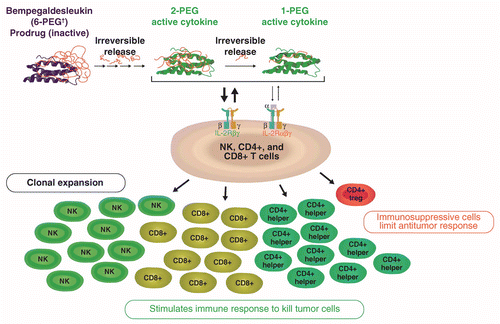
Bempegaldesleukin plus nivolumab in untreated, unresectable or metastatic melanoma: Phase III PIVOT IO 001 study design | Future Oncology

Nektar Therapeutics to Host Webcast Conference Call for Analysts & Investors Following Announcement of Update from Bristol-Myers Squibb and Nektar on the PIVOT-IO-001 Phase 3 Trial | BioSpace

785O PIVOT IO 001: First disclosure of efficacy and safety of bempegaldesleukin (BEMPEG) plus nivolumab (NIVO) vs NIVO monotherapy in advanced melanoma (MEL) | Request PDF

A systematic review of interleukin-2-based immunotherapies in clinical trials for cancer and autoimmune diseases - eBioMedicine

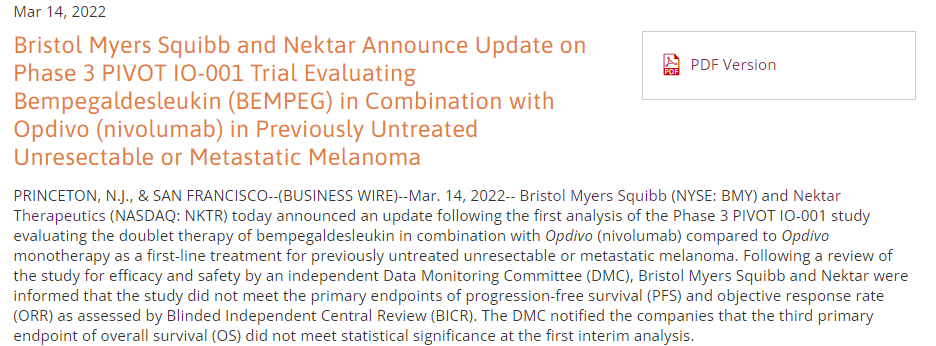
Bristol Myers Squibb and Nektar Announce Update on Phase 3 PIVOT IO-001 Trial Evaluating Bempegaldesleukin (BEMPEG) in Combination with Opdivo (nivolumab) in Previously Untreated Unresectable or Metastatic Melanoma | Business Wire

CONSORT diagram. a The PIVOT IO 001 study did not meet its primary end... | Download Scientific Diagram

Hans Hammers on X: "Quite disappointing for the field and concerning for respective renal / bladder efforts in the field. Question is why. Wrong drug ? Wrong therapeutic approach in general or

Bempegaldesleukin Plus Nivolumab in Untreated Advanced Melanoma: The Open-Label, Phase III PIVOT IO 001 Trial Results
![PDF] Bempegaldesleukin (NKTR-214) plus Nivolumab in Patients with Advanced Solid Tumors: Phase I Dose-Escalation Study of Safety, Efficacy, and Immune Activation (PIVOT-02). | Semantic Scholar PDF] Bempegaldesleukin (NKTR-214) plus Nivolumab in Patients with Advanced Solid Tumors: Phase I Dose-Escalation Study of Safety, Efficacy, and Immune Activation (PIVOT-02). | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/d6d60312b8c68ba838fc7b159401d5839051ca12/5-Table2-1.png)
PDF] Bempegaldesleukin (NKTR-214) plus Nivolumab in Patients with Advanced Solid Tumors: Phase I Dose-Escalation Study of Safety, Efficacy, and Immune Activation (PIVOT-02). | Semantic Scholar

ESMO: Adding bempeg to Opdivo lowered response rate in Bristol Myers-Nektar failed cancer trial | Fierce Biotech

Dr. Jennifer McQuade on X: "Dr Adi Diab of @MDAndersonNews @CancerMedMDA presenting updated data on #BEMPEG (NKTR-214) + Nivo in 1L #melanoma at #SITC2019 ORR 53%, PFS NR @ 18 mo f/u.