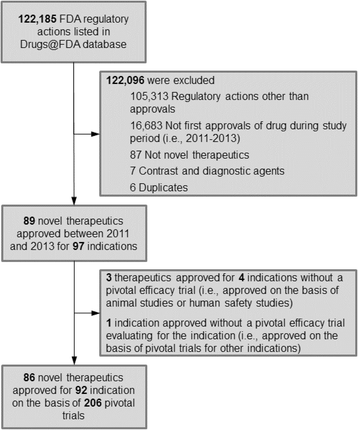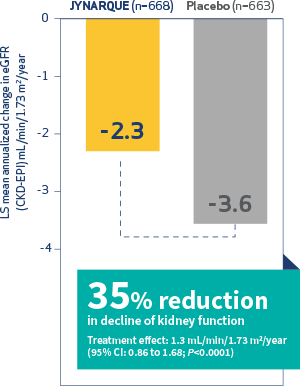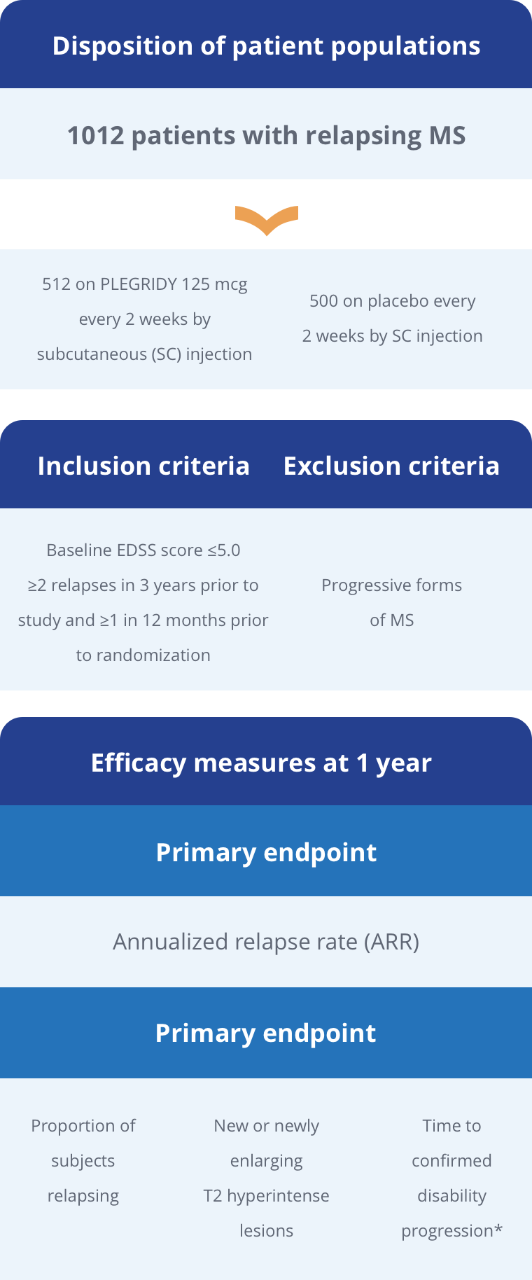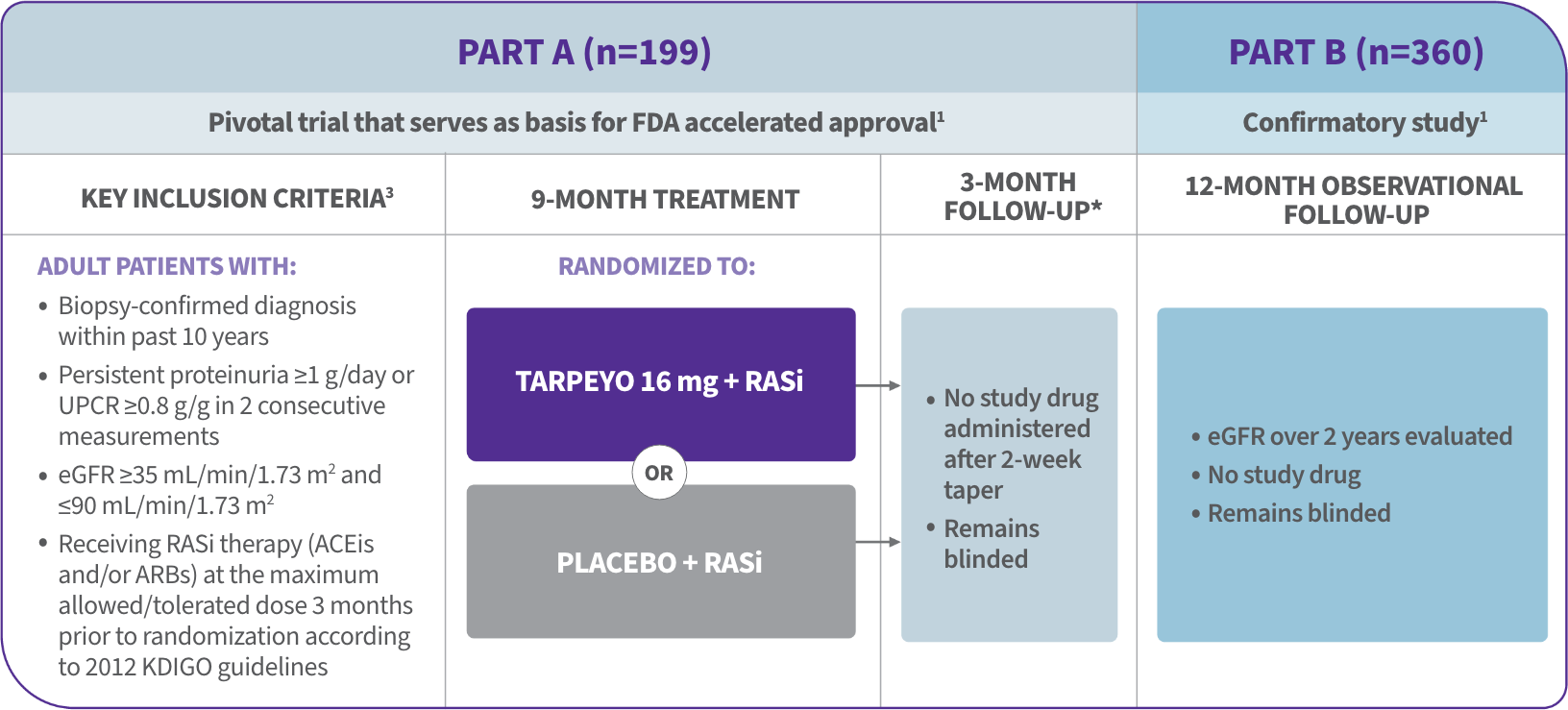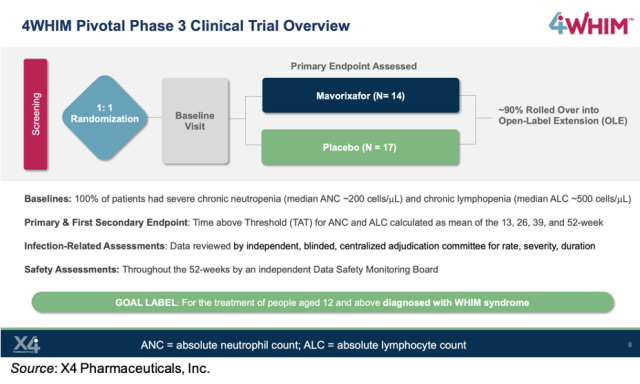
TACT Pivotal Clinical Trial of TULSA-PRO®: All Primary Efficacy and Safety Endpoints, and Key Secondary Endpoints Achieved | Profound Medical

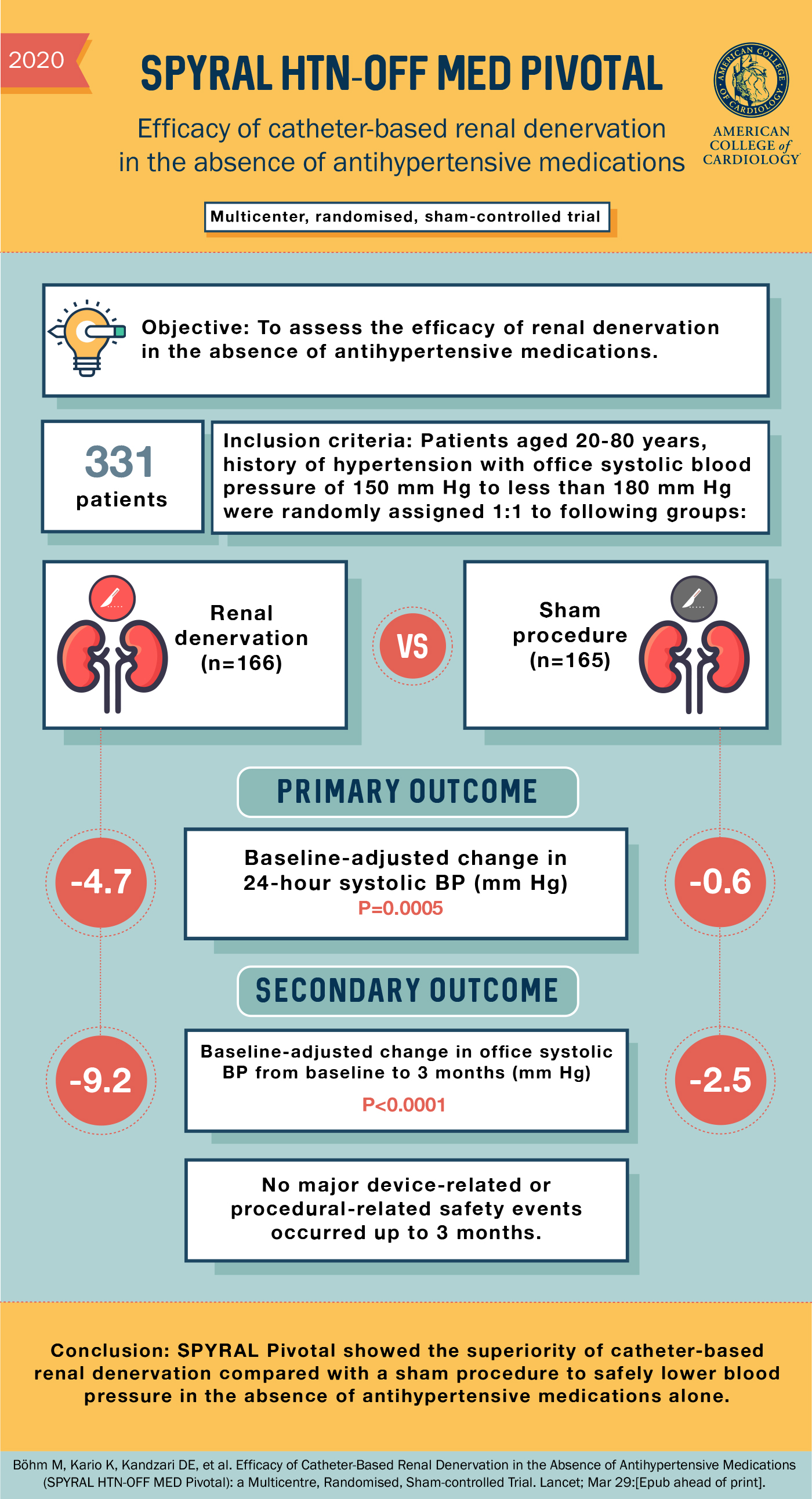
Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled trial - The Lancet
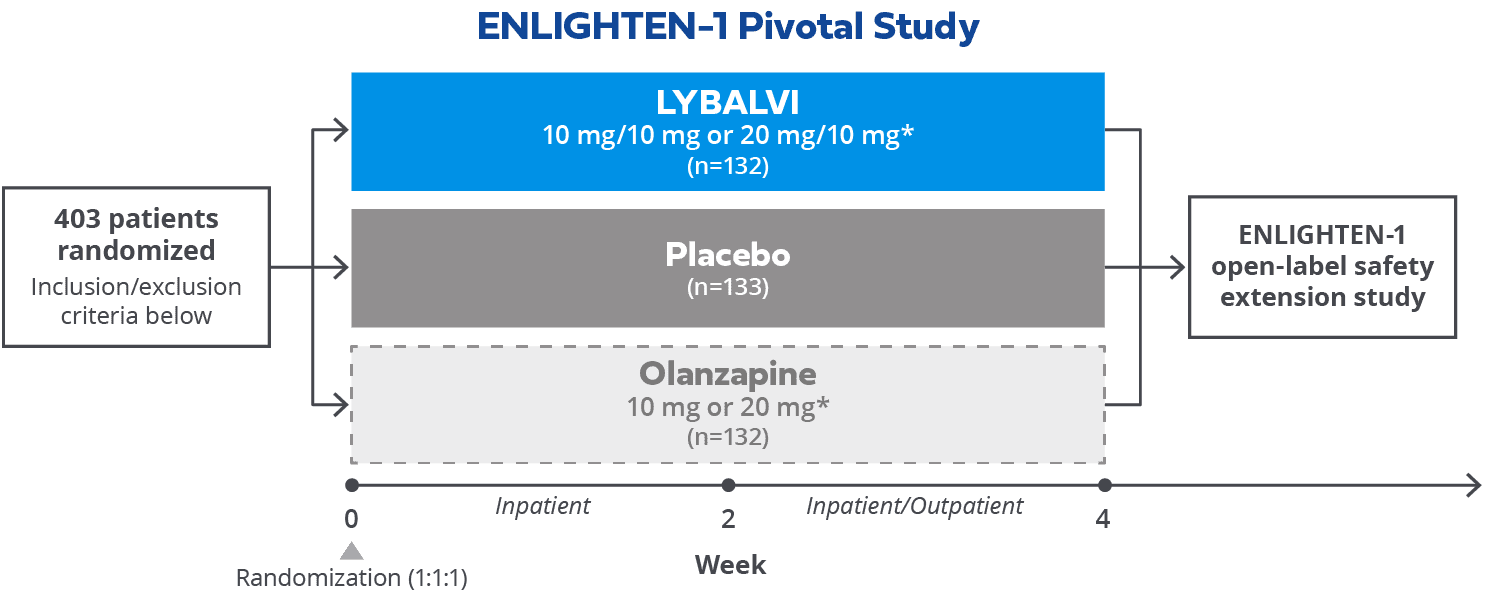
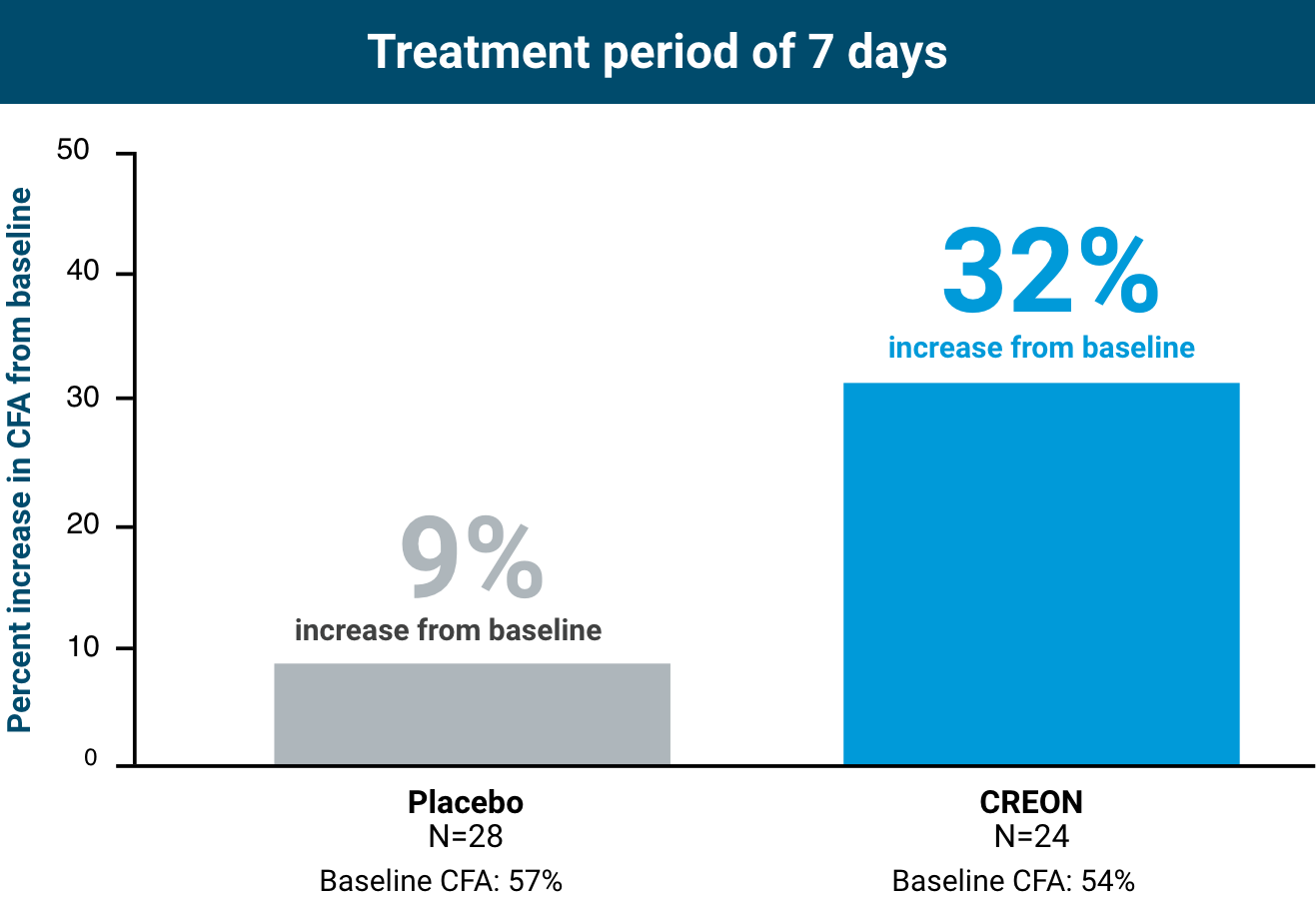
Review the Efficacy and Safety of LYBALVI® (olanzapine and samidorphan) from ENLIGHTEN-1 | Official Website for Healthcare Professionals

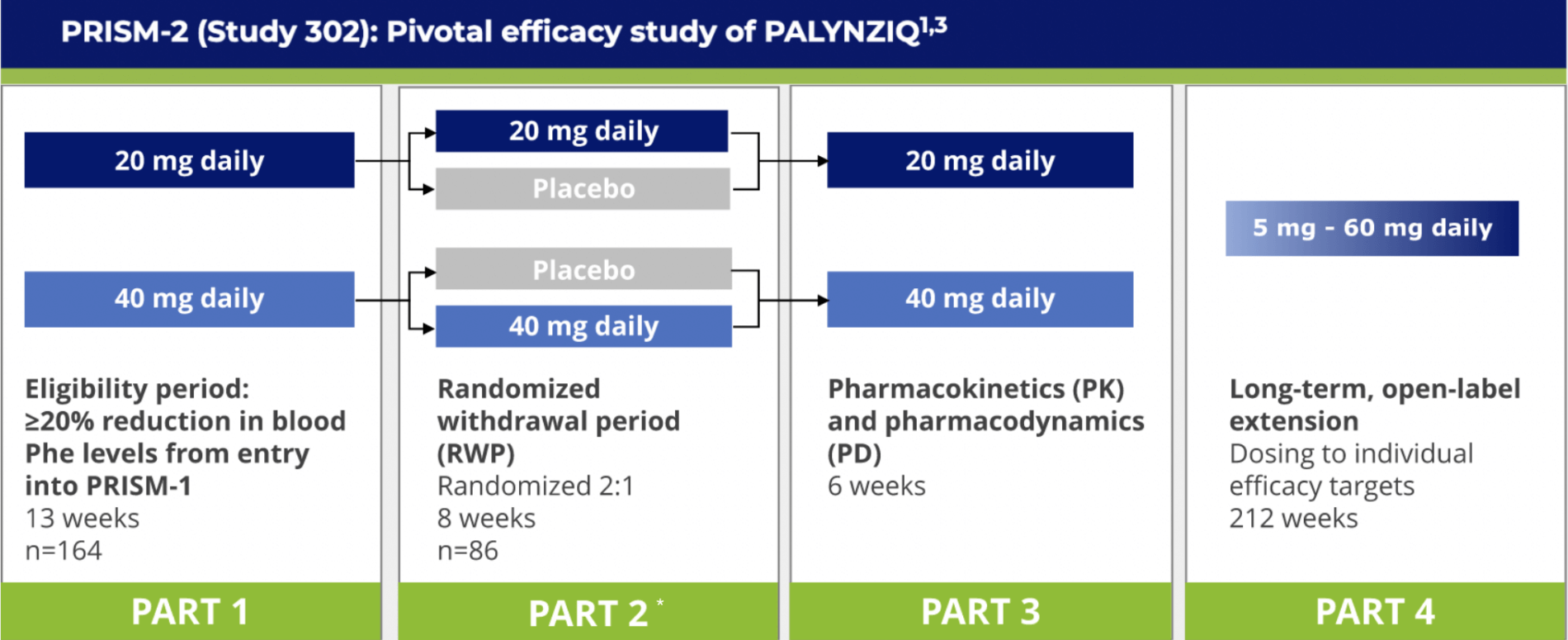
Study design, including the phase 3 pivotal trials (AD-301, AD-302) and... | Download Scientific Diagram